Which Element Has the Greatest Electronegativity N P as
Oxygen has 8 protons in the nucleus while nitrogen only has 7. As has an electronegativity of 22.
Electronegativity increases across a Period an horizontal row of the Periodic Table from left to right as we face the Table and DECREASES down a Group a column of the Periodic.

. See answer 1 Best Answer. The electronegativity chart describes how atoms can attract a pair of electrons to itself by looking at the periodic table you can identify and determine electronegativity values. And in fact the reason why theyre capable is hydrogen bonds is because.
Thus nitrogens N electronegativity is greater than that of. N P Si Na Lowest Electronegativity Greatest Electronegativity 5. The higher the value of the electronegativity the more strongly that element attracts the shared electrons.
According to Linus paulings electronegativity scale the Electronegativity of hydrogen is 220 and that of. Answer 1 of 2. P-N-P N-P-N P-N-P-N comparison between scr bjt and mosfet Check the related link for further information.
Which Element Has the Greatest Electronegativity N P as. Which element has the greatest electronegativity. Which of the following elements has the greatest electronegativity.
A bonding pair will experience. What 3 elements have the highest electronegativity. 118 rows The chemical elements of the periodic chart sorted by.
Which group has elements with the valence electron configuration of nsnp where n is the period number. Chlorine also a halogen. Arrange the following elements by increasing electronegativity.
This Table Shows Were Golds Electronegativity Element Project Periodic Table Diagram. Water molecule has covalent bond and due to the electronegativity of oxygen atom it will attract the bond pair of electrons towards itself and carry the partially negative charge and hydrogen. Oxygen Germanium Phosphorus Sulfur.
Which elements have the lowest and highest first. Click hereto get an answer to your question Which element has the greatest electronegativity. Of course they are made of sillicon.
N has an electronegativity of 30. On the Pauling scale the electronegativity of nitrogen and oxygen are respectively 30 and 35. Chlorine has a great attraction for electrons in a chemical bond.
Join Login Class 11 Chemistry Classification of Elements and Periodicity in. Nitrogen has the greatest electronegativity after Pauling. The halogens contain the most electronegative element F.
Electronegativity increases from bottom to top in groups and increases from left to right across periods. 103 rows Electronegativity Pauling scale The electronegativity of francium was chosen by. The reasons are listed below.
And those three elements are fluorine oxygen and nitrogen. Which of the following elements has the. Identify Which of the.
Electronegativity of element is the ability to attract the bonding electrons. P has an electronegativity of 22. Phosphorus has higher electronegativity than aluminium.
Arrange the following elements. Atomic number - Name alphabetically. The chemical symbols must be written with first letter capital.
Thus fluorine is the most electronegative element while francium is one of the least. 119 rows Electronegativity is a chemical property which describes how well an. Chlorine has the greatest electronegativity because out of all the choices it lies farthest to the right and top of periodic table.
The first scale of measuring electronegativity was given by Linus Pauling in 1932.
Which Group On The Periodic Table Is The Most Electronegative Quora

Electronegativity Chart Click To Download Free Pdf

The Electronegativity Of Lithium Is 1 0 Lesson Plans Chemistry Lesson
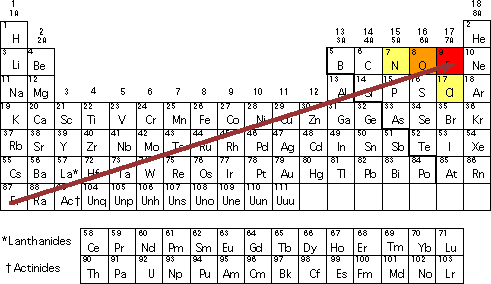

Comments
Post a Comment